List of members |
Facilities |
Internships and jobs |
PhD |
Publications |
News |
Team
- Permanent members: Gregory Cabailh, Stéphane Chenot, Fabio Finocchi, Jacek Goniakowski, Stéphane Guilet, Rémi Lazzari, Slavica Stankic
- Emeritus researchers: Jacques Jupille, Claudine Noguera
Metal-oxide interfaces are used in a number of applications due to the variety of their electronic, reaction or optical properties. Adhesion, (de)wetting, equilibrium form, stress, epitaxy, electronic structure and charge transfer, band alignment, electrical resistivity, interface chemistry, growth mode, role of defects are fundamental scientific properties which are questioned and whose understanding requires a global approach. Our strategy is based on a close coupling between atomistic simulations (ab initio and molecular dynamics) and experiments from the first stages of interface growth to the formation of percolated films and their dewetting. Our experimental approach is based on an original combination that has been seldom used in surface science: plasmonics for the study of growth and wetting modes, structural determination by EXAFS and surface analysis (photoemission, TPD, near field microscopy). Our studies are mainly motivated by adhesion issues in glass coatings (St-Gobain) and during the galvanization of steel sheets (ArcelorMittal).
Tuning Adhesion at Metal/Oxide Interfaces
The control of adhesion at metal/oxide interfaces responds to the concerns of the metallurgical industry, where surface oxides adversely affect the adhesion of zinc during galvanization on steel grades enriched in Al, Si, or Mn. For the zinc/alumina model interface, we predicted through ab initio calculations that a metallic buffer layer produces a strengthening of adhesion. However, since the oxidation of this layer is detrimental to adhesion, the use of multi-component thin films must allow control of the selective oxidation of the components and the corresponding oxide segregation. For this we have developed an effective method which, based on the characteristics of individual interfaces (estimated at the ab initio level), makes it possible to determine the equilibrium spatial distribution of the various components (oxide or metal) in the buffer layer, and to identify the least adhesive interfaces. In parallel, a joint experience/theory study has shown that the prior hydroxylation of alumina allows to switch from adhesive to cohesive rupture. Adhesion strengthening is due to the formation of ZnO entities dispersed at the metal/oxide interface during the desorption of excess hydrogen, the sub-coordinated anions of which form strong metal-oxygen bonds with the metal as well as with the oxide. The metal/hydroxyl reaction is thermally activated.
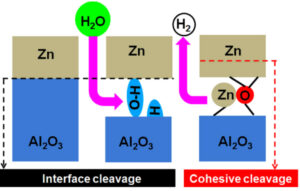
Figure 1: Effect of surface hydroxylation on Zn/alumina adhesion.
Publications
- H-L Thi Le, R. Lazzari, J. Goniakowski, R. Cavallotti, S. Chenot, C. Noguera, J. Jupille, A. Koltsov, and J.-M. Mataigne, Tuning Adhesion at Metal/Oxide Interfaces by Surface Hydroxylation, J. Phys. Chem. C 121 (2017) 11464 https://pubs.acs.org/doi/abs/10.1021/acs.jpcc.7b02456 https://hal.archives-ouvertes.fr/INSP/hal-01520408v1
- H-L Thi Le, J. Goniakowski, C. Noguera, A. Koltsov, and J.-M. Mataigne, J. Phys. Chem. C 121 (2017) 25143 https://pubs.acs.org/doi/10.1021/acs.jpcc.7b07112 https://hal.archives-ouvertes.fr/MATISSE/hal-01651879
librium shape and stress: Which dominates? The surface or the interface?
The shape of supported metallic nanoparticles drives their properties, the most common example coming from the field of heterogeneous catalysis. However, the respective contributions of interface energetics, stress, epitaxy and charge transfer can be relatively subtle for an oxide substrate. In the case of Ag nanoparticles, nanoplasmonics and EXAFS measurements were combined to establish the link between morphology, stress and adhesion variations. Molecular dynamics simulations of the equilibrium shape and the comparison with single crystal substrates which lead to a positive [MgO (001)] or negative [Al2O3(0001)] lattice parameter mismatch allowed us to determine the critical size of the nanoparticles from which the lattice parameter variation is no longer controlled by the substrate but only by surface stress according to the Laplace pressure. This work has made it possible to rationalise the crucial question of the size dependence of the energetics of supported Ag particles.
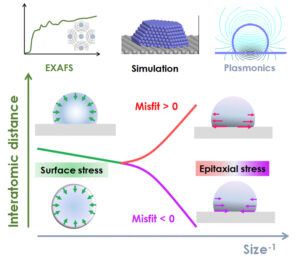
Figure 2: Stress regimes for a supported particle as a function of the inverse of its size and the parametric mismatch with the substrate.
Publication
- Rémi Lazzari, Jacek Goniakowski, Gregory Cabailh, Rémi Cavallotti, Nicolas Trcera, et al.. Surface and Epitaxial Stresses on Supported Metal Clusters. Nano Letters, American Chemical Society, 2016, 16 (4), pp.2574 – 2579. ⟨10.1021/acs.nanolett.6b00143⟩. ⟨hal-01442816⟩
ZnO: an epitaxy controlled by polar step edges
Glazings with thermal control, also called low-emissivity or anti-solar, use silver films with thicknesses of approximately ten nanometers resulting in a transparent film in the visible but reflective in the infrared range. To stabilise silver with respect to dewetting which is deleterious for the desired functionality, the metal is deposited by sputtering within complex stacks in direct contact with textured films of ZnO (0001). Our past studies on single crystals have shown the existence of a hexagon/hexagon Ag (111)/ZnO (0001) epitaxial relationship with high parameter mismatch. The latter goes against the existence of a 30° rotated orientation for which the disagreement is much weaker. By analysing deposits on the ZnO rhombohedral face, we have shown that nucleation and crystallographic orientation are in fact driven by the polar step edges. Plasmonics, STM, LEED and ab initiocalculations confirm anisotropic growth and a clear preference for the O terminated step edge, compared to the Zn one or to the non-polar step edge formed by anions and cations.
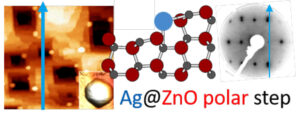
Figure 3: Preferential nucleation of Ag islands on ZnO polar edges.
Publication
- S. Benedetti, I. Valenti, S. Valeri, S. Castilla, E. Touzé, Y. Bronstein, A. Toumar, F. Finocchi, R. Lazzari. Polar Step-Driven Metal Nucleation and Growth: The Ag/ZnO(101̅0) Case. J. Phys. Chem. C 124 (2020) 6130 https://pubs.acs.org/doi/10.1021/acs.jpcc.9b11464
Photoemission and electric contact at the Ag/ZnO interface
The potential use of metallic thin films as transparent conductive layers to replace indium tin oxide requires to control the nature of the electrical contact (resistive or Schottky), at the metal/oxide interface. On the ZnO surfaces, there is a great disparity in the literature depending on the substrate preparation. In this context, we undertook a systematic in situ study of the band alignment at the interface Ag/polar ZnO(0001) surfaces, as a function of the termination and the hydrogenation state. Surface chemistry, work function variation, band bending and charge transfer were investigated. The originality of the study was to combine laboratory photoemission techniques (XPS/UPS) and high-energy measurements (HAXPES) at the synchrotron to determine the alignment between the crystal bulk and its extreme surface. While hydrogenation strongly modulates the position of surface energy levels, a value independent of surface preparation has been found for the Schottky barrier.
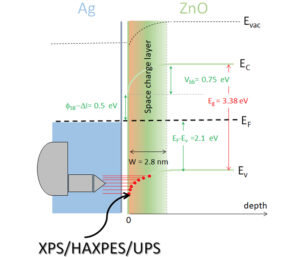
Figure 4: Band alignment diagram at the Ag/ZnO (0001) interface deduced from the photoemission analysis at different energies.
Publication
- Ekaterina Chernysheva, Waked Srour, Bertrand Philippe, Bulent Baris, Stéphane Chenot, et al.. Band alignment at Ag/ZnO(0001) interfaces: A combined soft and hard x-ray photoemission study. Physical Review B: Condensed Matter and Materials Physics (1998-2015), American Physical Society, 2018, 97 (23), pp.235430. ⟨10.1103/PhysRevB.97.235430⟩. ⟨hal-01839974⟩
Solid state dewetting: friend or foe?
Solid-state dewetting of polycrystalline films impairs the functionality of metallic coatings which are often obtained in a metastable state. The study in real time of the dynamics of the Ag/SiO2 case by environmental scanning electron microscopy allowed us to clearly highlight three distinct regimes: induction with grain boundary grooving, propagation of holes, then sintering. The final particles result from a high selectivity of some grains in the layer induced by the diffusion before dewetting. Oxygen accelerates all the processes, but a detailed analysis of the curvature of the dewetting front shows that a purely capillary approach to the phenomenon does not describe the experimental observations. There is no spread of a rim but rather a growth and narrowing of certain grains.
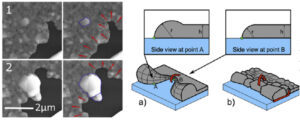
Figure 5: Scanning microscopy images extracted from a video showing the importance of grains in dewetting. (right) “Capillary” and “granular” visions of dewetting.
However, it is possible to take advantage of solid-state dewetting to synthesise nanostructures the size and periodicity of which can be controlled by surface structuring. The latter is obtained by nano-imprint patterning of silica gels. The resulting particle networks have specific optical properties, due to diamond shape particles, but also to a large-scale organisation and periodicity (“surface lattice resonance”). The synthesis method makes it possible to obtain centimetre-size samples on transparent substrates.
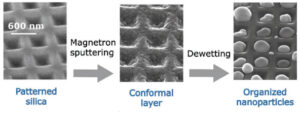
Figure 6: Synthesis of arrays of metallic Ag particles by dewetting on waffle-patterned substrates (nanoimprint).
Publications
- P Jacquet, Renaud Podor, J Ravaux, J Teisseire, I Gozhyk, et al.. Grain growth: The key to understand solid-state dewetting of silver thin films. Scripta Materialia, Elsevier, 2016, pp.128-132. ⟨10.1016/j.scriptamat.2016.01.005⟩. ⟨hal-01274691⟩
- P. Jacquet, Renaud Podor, J. Ravaux, J. Lautru, J. Teisseire, et al.. On the solid-state dewetting of polycrystalline thin films: Capillary versus grain growth approach. Acta Materialia, Elsevier, 2018, 143, pp.281-290. ⟨10.1016/j.actamat.2017.08.070⟩. ⟨hal-01996081⟩
- Paul Jacquet, Barbara Bouteille, Romain Dezert, Joseph Lautru, Renaud Podor, et al.. Periodic Arrays of Diamond‐Shaped Silver Nanoparticles: From Scalable Fabrication by Template‐Assisted Solid‐State Dewetting to Tunable Optical Properties. Advanced Functional Materials, Wiley, 2019, pp.1901119. ⟨10.1002/adfm.201901119⟩. ⟨hal-02159208⟩
Nanoplasmonics: from growth to charge transfer
Nanoplasmonics has proven to be a very relevant tool for the in situ study of the growth, equilibrium form and wetting of metallic nanoparticles on oxide substrates. Through dielectric simulations of UV-vis spectra taking into account the effects of shape, electrostatic interactions, temperature and inhomogeneous broadening, it was possible to isolate, in the case of silver, the nucleation/growth/coalescence phases on the evolution of size, density and aspect ratio. An inversion algorithm of optical spectra in polarisation allowed us to demonstrate, for a given shape, the universality of the resonant modes involved, whatever the metal (Ag, Au, Zn) or the substrate (insulator or semiconductor). The fundamental role of the energy position of the inter-band transitions of the metal was thus identified in the observation of the resonances. Finally, although gold nanoparticles are usually stabilised in solution by thiolated organic molecules, the role of the ligand on the plasmon resonance of the metallic core has not been much explored so far. By combining UV-vis spectroscopy, ellipsometry on self-assembled layers and dielectric simulations, we have demonstrated that the aromatic thiol groups compared to the aliphatics lead to a significant and quantifiable charge transfer towards the d bands of gold giving rise to an unexpected shift of the plasmon resonance.
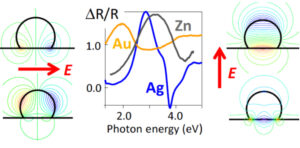
Figure 7: Polarization plasmonic eigenmodes and differential reflectance spectra of metals/alumina.
Publications
- R. Lazzari, J. Jupille, R. Cavallotti, I. Simonsen,J. Growth kinetics and size-dependent wetting of Ag/α-Al2O3(0001) nanoparticles studied via the plasmonic response. Phys. Chem. C 23 (2013) 135707 https://iopscience.iop.org/article/10.1088/0957-4484/23/13/135707
- Rémi Lazzari, Jacques Jupille, Rémi Cavallotti, Ingve Simonsen. Model-Free Unraveling of Supported Nanoparticles Plasmon Resonance Modes. Journal of Physical Chemistry C, American Chemical Society, 2014, 118 (13), pp.7032 – 7048. ⟨10.1021/jp500675h⟩. ⟨hal-01442823⟩
- Claire Goldmann, Rémi Lazzari, Xavier Paquez, Cédric Boissière, François Ribot, et al.. Charge Transfer at Hybrid Interfaces: Plasmonics of Aromatic Thiol-Capped Gold Nanoparticles. ACS Nano, American Chemical Society, 2015, 9 (7), pp.7572-7582. ⟨10.1021/acsnano.5b02864⟩. ⟨hal-01291241⟩
How chromium adsorption reveals persistent hydroxylation of the α-Al2O3(0001) surface?
The ubiquitous presence of oxides and water in the environment has prompted over the last few decades a wealth of studies on hydration/hydroxylation of reference surfaces. However, puzzling issues resist analysis. To lift the persistent blur on the widely studied vacuum annealed α-Al2O3(0001) surface – is it bare or hydroxylated? – a Cr deposit was examined by absorption spectroscopy (EXAFS and XANES) and photoemission experiments, supported by density functional theory calculations. The thermodynamically stable Cr3+-O2H/alumina configuration, highlighted via Cr K edge analysis, unambiguously demonstrates the presence of surface OH groups and questions the known α-Al2O3(0001) surface structure. This study is emblematic of the topicsoverlap involved in the study of metal/oxide interfaces.
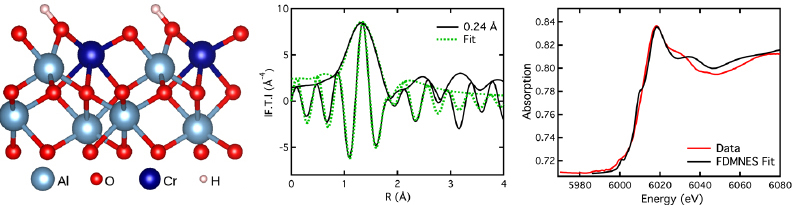
Figure 8: Cr absorption configuration involving surface hydroxylation which allows to reproduce experimental XAS spectra.
Publication
- Maya Messaykeh, Jacek Goniakowski, Gregory Cabailh, Jacques Jupille, Rémi Lazzari, et al.. Chromium Adsorption Reveals a Persistent Hydroxylation of Vacuum-Annealed α-Al 2 O 3 (0001). Journal of Physical Chemistry C, American Chemical Society, 2019, 123 (48), pp.29245-29254. ⟨10.1021/acs.jpcc.9b08907⟩. ⟨hal-02452290⟩
Collaborations
Industries
- ArcelorMittal Maizières Research, France
- Saint-Gobain Recherche, France
Academia
- Institut des Sciences Moléculaires d’Orsay, France
- Synchrotron SOLEIL, France
- Universita di Modena e Reggio Emilia, Italy
- Norwegian University of Science and Technology, Norway
- Aachen University, Germany
Funding
- CIFRE thesis