Quantum transition: when deuterium breaks the symmetry
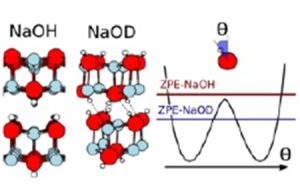 Sodium hydroxide (NaOH), (NaOH), does not exhibit any phase transition at low temperatures. By replacing hydrogen with deuterium (D), a transition appears at 153K between two distinct crystal structures, with a strong expansion (>5%). At room temperature, the structures of NaOD and NaOH become very similar. This spectacular transition induced by a simple isotopic exchange was detected in 1985 and had never been explained: generally, in condensed matter, nuclei are considered classical objects (unlike electrons, naturally regarded as quantum). In this context, statistical properties are insensitive to isotopes. INSP researchers managed to elucidate this phenomenon, using a quantum description of the nuclei.
Sodium hydroxide (NaOH), (NaOH), does not exhibit any phase transition at low temperatures. By replacing hydrogen with deuterium (D), a transition appears at 153K between two distinct crystal structures, with a strong expansion (>5%). At room temperature, the structures of NaOD and NaOH become very similar. This spectacular transition induced by a simple isotopic exchange was detected in 1985 and had never been explained: generally, in condensed matter, nuclei are considered classical objects (unlike electrons, naturally regarded as quantum). In this context, statistical properties are insensitive to isotopes. INSP researchers managed to elucidate this phenomenon, using a quantum description of the nuclei.
Caption
The NaOH/D structure. In NaOH, quantum fluctuations prohibit the formation of hydrogen bonds and the OH groups oscillate around the vertical. Deuterium, although chemically equivalent, is heavier and therefore «less quantum». Its zero point energy (ZPE) is lower than the barrier, the OD groups are trapped in one of the potential wells and form hydrogen bonds which ensure the cohesion of the crystal… until thermal fluctuations take over!
Reference
“When Quantum Fluctuations Meet Structural Instabilities: The Isotope- and Pressure-Induced Phase Transition in the Quantum Paraelectric NaOH”
S. Schaack, E. Mangaud, E. Fallacara, S. Huppert, P. Depondt and F. Finocchi
Physical Review Letters, 131, 126101 (2023)
Contacts
- Fabio Finocchi : fabio.finocchi(at)insp.jussieu.fr
- Philippe Depondt : depondt(at)insp.jussieu.fr,
- Simon Huppert : simon.huppert(at)insp.upmc.fr