List of members |
Facilities |
Internships and jobs |
PhD |
Publications |
News |
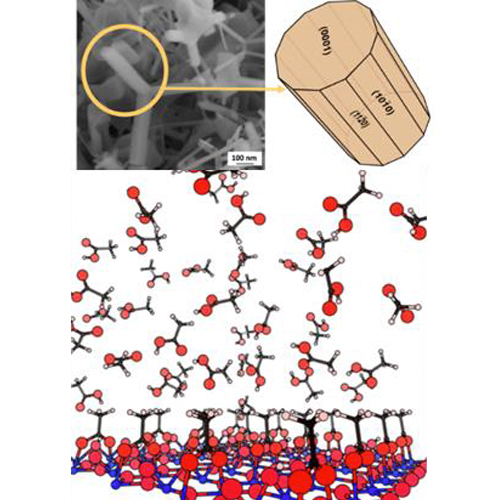
Founded in 2002, this team brings together experimentalists and theoreticians interested in the effects of dimension reduction in oxide materials. Thanks to a joint experience/theory collaboration, the group has developed over the past years a recognized expertise in the science of oxide surfaces (surfaces, thin films, nano-objects). Its activity is based on long-term collaborations with other universities, national and international teams, but also with research centers of large industrial groups (Saint-Gobain and ArcelorMittal). The team develops a limited number of perennial scientific themes: atomic and electronic structure, nature of defects, polarity, reactivity, adhesion, hydroxylation, dissolution/precipitation, metal/oxide interfaces. They are mainly focused on binary oxides in various forms (crystals, thin films, nanopowders), in environments ranging from ultra-high-vacuum to liquids or in contact with metals.
4 topics
News and events
- October 2023
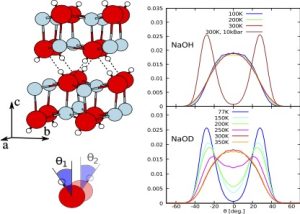 |
Latest publication: The puzzling isotope-induced quantum phase transition in sodium hydroxide
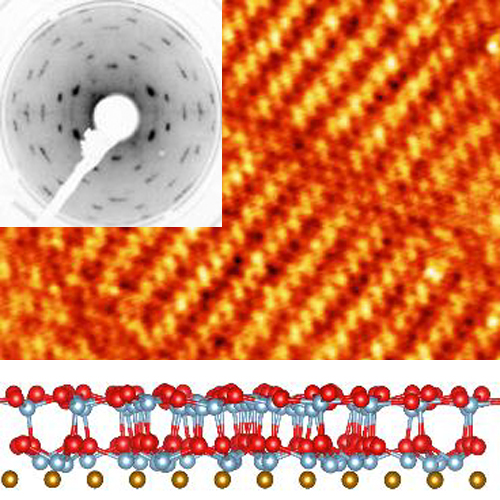
Anhydrous Sodium Hydroxide (NaOH) has a simple structure, from a few K up to room temperature. In contrast, NaOD shows a structural phase transition at 153 K. This spectacular isotope-induced phase transition has been discovered in 1986 and theoretically explained by our group for the first time, by using fully quantum simulations. |
- Septembre 2023
| Congratulations to Ramiro Zapata for the “Michel CANTAREL” bourse of the Société Française du Vide (SFV) at PLATHINIUM 2023 and for the prize for the best oral presentation @ C’NANO 2023. |  |
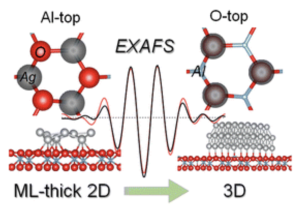 |
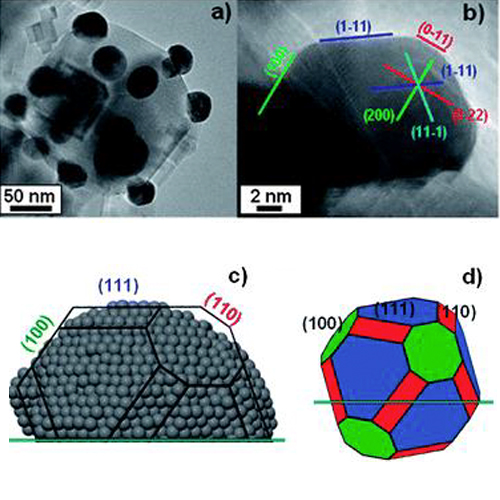
Publication of our work investigating the atomic structure of the Ag/α-Al2O3(0001) interface using X-ray absorption spectroscopy and numerical simulations on nanoscale clusters, revealing size-dependent changes in Ag cluster dimensions and coordination. The study shows that as Ag coverage increases, 2D clusters transition to 3D islands, accompanied by reduced adhesion energy at the Ag/α-Al2O3(0001) interface. |