List of members |
Facilities |
Internships and jobs |
PhD |
Publications |
News |
Team
- Permanent members: Gregory Cabailh, Stéphane Chenot, Fabio Finocchi, Jacek Goniakowski, Stéphane Guilet , Rémi Lazzari, Anna Levy, Slavica Stankic
- Emeritus researchers: Jacques Jupille, Claudine Noguera
The reaction behaviour of oxide surfaces is often governed by the nature of defects (vacancies, kinks, steps, etc...), the control and characterisation of which require specific samples (powders, thin films, bulk crystals) adapted to each measurement or question. Beyond their respective advantages, their comparison is extremely fruitful.
Noteworthy advances in the role of facets and environment were obtained in the group by combining (a) infrared spectroscopy on simple binary (MgO, ZnO) or mixed oxide nanopowders at pressures ranging from ultra-high vacuum (a strong originality of the team experiments) to conditions close to normal and (b) atomistic simulations. The specific reactivity of nanopowders has also been exploited in the context of potential antibacterial/antiviral medical applications in liquid biological medium.
Titanium dioxide is the archetype of oxides the reactivity of which, in particular in photo-catalysis, is driven by the nature of the polymorphs and of the defects present in the surface region. The group recently developed an original approach on charge carriers in rutile, around the technique of electron energy loss, with unprecedented results on the origin, nature, polaronic character, distribution and transport of these carriers.
Identification of ZnO nanopowders facets during water adsorption
The acid-base properties and the surface reactivity of oxides strongly depend on the crystallographic orientation. In the case of ZnO, if the extended single crystalsurfaces make it possible to address these questions, the fumes of ZnO with well-defined facets can be seen as single crystals with multiple facets simultaneously involving all the low index orientations, (101̅0),(112̅0),(0001),(0001̅). On such a powder in which polar orientations represent only 25% of the total surface, we have shown that the adsorption of water can serve as a probe to specifically identify each facet. In particular, the monitoring by infrared spectroscopy of the exposure from ultra-vacuum-high up to high pressures and the ab initiosimulation enabled: (i) the analysis of the very first stages of adsorption, (ii) the identification for the first time of the hydroxylation configurations on the facet (112̅0) and (iii) new water structures on the(101̅0) surface.
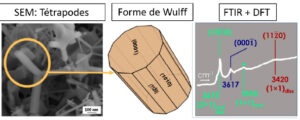
Figure 1: The morphology of ZnO fumes reproduces the Wulff construction involving the facets. The OH bands are associated by calculation with specific configurations on each facet.
Publication
- F. Haque, S. Chenot, F. Viñes, F. Illas, S. Stankic, J. Jupille, ZnO powders as multi-facet single crystals, Phys. Chem. Chem. Phys. 19 (2017) 10622 https://doi.org/10.1039/C7CP01635B https://hal.sorbonne-universite.fr/hal-01518347
Probing the specific reactivity of MgO surface sites with H2O and H2
MgO powders with large specific surface appear to be a model system particularly well suited to study the relationship between surface structure and reactivity. The cubic equilibrium shape could suggest that the surface only involves (100) facets. However, a more realistic view of the surface of these highly dispersed materials implies a considerable concentration of highly reactive defects such as steps, corners and point defects (vacancies/ anionic/cationic antisites, interstitial sites). The selective dissociation of H2 at these sites and the concomitant formation of hydroxyl and hydride groups was demonstrated by FTIR spectroscopy. However, the preparationand analysis under ultra-high-vacuum gives access to spectra with greater information than those in the literature. Along with the identification of the most thermodynamically stable surface structures -which irreversibly dissociate the H2 molecules in the low pressure regime (up to 10-3 mbar) -the measurements also demonstrated that the population of distant defect sites results in cooperative adsorption of more than one H2molecule. Indeed, theory has proven that dissociation becomes kinetically hindered whenever the H+/H− adsorption sites are not neighbouring -regardless of the stability of the corresponding adsorption structure. Consequently, adsorption on MgO has been described with hybrid surface structures –a combination of sites with different reactivity and stability -on which co-adsorption has proven to be essential to populate in H+/H− defective sites spatially distant. During the dissociation of water on MgO powders of different specific surfaces and having different synthesis histories, calculations identified the signatures of the hydroxyl groups on the corners, the monoatomic steps, the kinks and the vacancies on monoatomic steps, reproducing the experimental results both qualitatively and quantitatively.
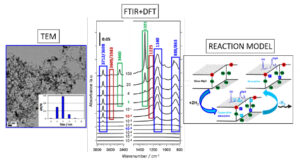
Figure 2: The 15nm size nanocubes imaged in TEM prove to be perfect candidates for infrared studies of hydrogen and water absorption. The ultra-high-vacuum measurements demonstrate the richness of the sites involved which requires the use of calculations to rationalise it.
Publications
F. Haque, F. Finocchi, S. Chenot, J. Jupille, S. Stankic , Interplay between Single and Cooperative H2 Adsorption in the Saturation of Defect Sites at MgO Nanocubes, J. Phys. Chem. C 122 (2018) 177387 https://doi.org/10.1021/acs.jpcc.8b03192 https://hal.archives-ouvertes.fr/hal-01871185/
- F. Finocchi, F. Haque, S. Chenot, J. Jupille, S. Stankic, Water dissociation on the low-coordinated sites of MgO nanopowders, J. Mat. Research, 34 (2019) 408 https://doi.org/10.1557/jmr.2018.461 https://hal.sorbonne-universite.fr/hal-02400798
The effects of water and cell culture media on ZnMgO nanoparticles
ZnMgO nanoparticles undergo profound morphological changes in aqueous solution. In a complex cell culture medium, the majority of nanoparticles agglomerate and are covered with surrounding proteins. Thus, mammalian cells encounter nanoparticles with contact surfaces altered by proteins. Thus, the cytotoxicity of ZnMgO strongly depends on the environment: in the presence of serum proteins, ZnMgO nanopowder has been shown to be harmless for mammalian cells while being highly toxic in a serum-free medium or a medium containing only albumin. In fact, it has been possible to demonstrate that nanostructured ZnMgO reaches living cells with a different morphology and surface structure compared to synthesized particles maintained under vacuum while its biocompatibility can be modified by proteins from the biological environment.
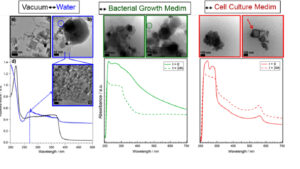
Figure 3: Effects of water and culture medium on the morphology (TEM), optical absorption (UV-vis) and cytotoxicity of ZnMgO nanopowders.
Publication
- J. Vidic, F. Haque, J.-M. Guigner, A. Vidy, C. Chevalier, S. Stankic, Effects of Water and Cell Culture Media on the Physicochemical Properties of ZnMgO Nanoparticles and Their Toxicity toward Mammalian Cells, Langmuir 30 (2014) 11366 https://doi.org/10.1021/la501479p
Structural identification of TiO2 polymorphs using laboratory photoemission
The photo-catalytic properties of titanium dioxide strongly depend on the nature of the polymorphs involved (rutile/anatase), but also on the crystallographic orientation of their facets. Their rapid identification encounters detection sensitivity issues, in particular in the case of thin layers, the emblematic example being their use in self-cleaning glazing. In this context, our group has demonstrated the interest of laboratory photoemission and in particular the Auger transitions involving the valence band. The LMV lines directly reflect the Ti 4sp-O 2p hybridisation of the valence band which are intrinsic to the polymorph and its orientation. The advantage compared to a UPS measurement is the lack of sensitivity to contamination of this intrinsic Ti Auger line. Reference spectra acquired on monocrystalline surfaces – rutile (110) and anatase (101) / (100) – allow quantitative fits of Auger line shapes in the case of mixed powders. In agreement with diffraction, the method allows the monitoring of the anatase-rutile transformation as a function of temperature of P25 powders.
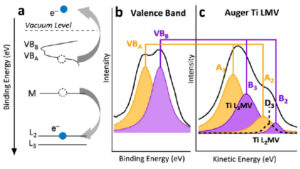
Figure 4: Correspondence between the shape of the valence band and that of the Auger LMV line in titanium dioxide.
Publication
- P. Borghetti, E. Meriggio, G. Rousse, G. Cabailh, R. Lazzari, J. Jupille, Photoemission Fingerprints for Structural Identification of Titanium Dioxide Surfaces ? J. Phys. Chem. Lett. 7 (2016) 3223 https://pubs.acs.org/doi/10.1021/acs.jpclett.6b01301 https://hal.archives-ouvertes.fr/hal-01362684
Origin and nature of bandgap states in TiO2
The complex physical chemistry on the surface of titanium dioxide is intimately linked to its reducible nature. The dominant defects of rutile are the bulk Ti interstitials and the O vacancies at the surface; they have been the subject of numerous studies on the (110) surface of rutile. They give rise to excess electrons which spectroscopically populate a Ti 3d type bandgap state and which are at the origin of the n-type conductivity. The study carried out within the group focused on two central questions which remain subject to controversies: (i) the respective role of the two types of defects in the bandgap state and (ii) the dual character of the excess electrons due to their polaronic nature or the coexistence of free and bound carriers. In an original way, these issues were tackled by high-resolution reflection electron energy loss measurements (HREELS) combined with dielectric simulations. Experimental approaches and specific sample treatments (surface heating by exposure to a filament, cooling, off-specular measurements, electron bombardment, exposure to O2 / H2O) were used to determine specific conditions for which vacancies or interstitials were the only type of surface defect contributing to the bandgap state. Both give rise to similar profilesof excess charges. The dielectric analysis of the effect on the HREELS spectra of the induced screening by the carriers and by the bandgap states during exposure to oxygen clearly demonstrated a dual behaviour of the carriers. On the other hand, surface and bulk defects have distinct transport behaviours.
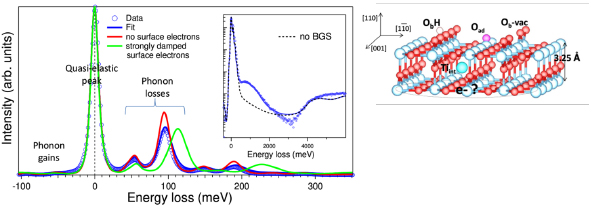
Figure 5: (Left) Dielectric simulation of (HR)EELS spectra in the phonon and bandgap states (inset) zones. (Right) Atomic model of the defective surface TiO2(110).
Publications
- J. Li, R. Lazzari, S. Chenot, J. Jupille, Contributions of oxygen vacancies and titanium interstitials to band-gap states of reduced titania? Phys. Rev. B 97 (2018) 041403(R) https://journals.aps.org/prb/abstract/10.1103/PhysRevB.97.041403 https://hal.sorbonne-universite.fr/hal-01700847
- R. Lazzari, J. Li, J. Jupille, Dielectric study of the interplay between charge carriers and electron energy losses in reduced titanium dioxide? Phys. Rev. B, 98 (2018) 075432 (2018) https://journals.aps.org/prb/abstract/10.1103/PhysRevB.98.075432 https://hal.sorbonne-universite.fr/hal-01872526
Collaborations
- University College of London,UK
- University of Manchester, UK
- Diamond Light Source, UK
- ESRF, France

