Team
- Permanent members: Emily Lamour, Sébastien Steydli, Stéphane Macé, Martino Trassinelli, Christophe Prigent, Dominique Vernhet
The interest of research and industrial communities for nanoparticles (NPs) is increasing thanks to their numerous interesting properties and technological applications. Gold colloids are usually produced by wet chemical reduction which enables the fabrication of monodispersed size distributions in a well-controlled manner. However, these methods involve capping agents to prevent the aggregation. This molecular capping can be particularly problematic since NPS are often used for their surface properties, highly sensitive to the chemical environment. In this frame, the PLAL method (pulsed laser ablation in liquid) is appealing for its versatility, simplicity and biocompatibility. Based on the simple laser irradiation of a solid sample in a liquid medium, NPs are produced through the creation of a plasma confined in a cavitation bubble where nucleation and coalescence processes occur. Using this method, no surfactant is needed to stabilize the colloid: the NPs carry a negative charge that prevents their agglomeration through electrostatic repulsion. This is of particular interest since it implies that the NP is purely composed of the original ablated material. Furthermore, their surface is entirely free for either a subsequent functionalization, a chemical reaction, or surface adsorption. These NPs are therefore not only interesting for various of applications but they can also be considered as model systems for fundamental studies investigating NP surface properties. Using such pure systems, theoretical modelling can be performed with confidence, and, experimental measurements can be interpreted trustworthy. The main drawback of PLAL is related to the size distributions that are usually bimodal (~10 and 100 nm). However, this polydispersity as well as the colloid stability can be further controlled by adding halide salts in the liquid medium. The underlying processes at the origin of the colloid stability are still matter of debate. This research project aims to answer to the following questions:
- Where does the negative charge, responsible of the colloid stability, come from?
- Are these nanoparticles really pure, even in the presence of halides?
- Using the PLAL versatility can we engineer the electronic properties of these NPs for the purpose of energy harvesting applications, I.e. using these NPs as plasmic photosenitizers.
Why colloids produced by PLAL are stable?
The colloid stability of PLAL NPs is attributed to an intrinsic net surface negative charge responsible for mutual electrostatic repulsion. However, its unknown origin is a matter of debate. Whether produced in pure or saline water, two main scenarios are investigated. The first one involves partial surface oxidation or halide adsorption (Hyp. 1 and 3) whereas the second one supposes that electrons are captured during the plasma phase of the ablation process (Hyp. 2).
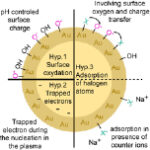
Figure 1: Surface composition and charge picture of PLAL-generated NPs.
To clarify this question, we perform x-ray photoelectron spectroscopy (XPS) measurements on PLAL NPs in order to investigate their surface composition.
- “Measurement protocol matters”. We have demonstrated in De Anda Villa et al. (Langmuir 35 (2019) 11859 – HAL), that the surface oxidation and chemical composition of these bare NPs can be affected by the chemical environment. Instead of probing them after drop-casting the colloid on a substrate in ambient conditions, our experiments are performed on a beam of free-standing NPs produced by an aerodynamic lens in order to avoid any substrate effect. The experiments are performed at the SOLEIL synchrotron facility on the PLEIADES beamline.
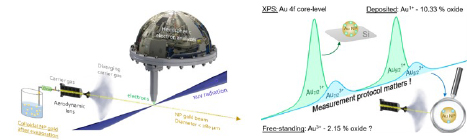
Figure 2: Experimental setup and illustration of the effect of the gold nanoparticle deposition on the surface oxidation.
- “Data analysis matters also”. XPS spectra fitting is intrinsically delicate due to the presence of multiple components, numerous unknowns and non-trivial background. This is especially the case when a multiple component overlap is expected and/or when a component is close to the noise level. In order to extract solid interpretations, we therefore apply a fitting procedure based on Bayesian statistics (Trassinelli, Nucl. Instrum. Methods Phys. Res. 408 (2017) 301 – HAL) which can, among other advantages, evaluate the relative probability of one fitting model compared to another one. The data analysis procedure applied is able to quantify the presence probability of the gold oxide component.
- “Halide ions are adsorbed on the NP surface”. Based on this robust procedure, the presence of halides on the NP surface has been confirmed (A. Lévy et al., to be published) through the comparison of XPS measurements of the halide core-level (Br 3d) recorded on pure saline water and saline water including gold NPs.
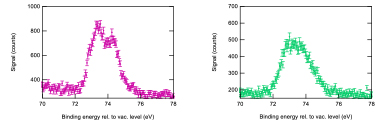
Figure 3: XPS Br 3d measurements on pure saline water and saline water containing gold NPs evidencing a clear signature of Br ions adsorbed on the NPs surface.
- “DFT calculations”. This project is strengthened by theoretical DFT calculations which evaluate the probability of halide adsorption through adsorption energy calculations. This theoretical study is performed for a model gold surface interacting with halide ions in different configurations such as: a surface containing OH–, O2– or Br– oxidation species or doped by a negatively charged Br atom. The preliminary results evidence the crucial role of the counterion (Na+) in this process.
Are colloids produced by PLAL pure?
One of the more striking property of the PLAL colloids is the high control of their surface and bulk chemical composition. The NP bulk composition is usually supposed to be solely made of the original material. However, in the case of NPs produced in halide saline water, is the NP doped by halide ions or their presence is limited to the surface? Such NP “doping” is not unrealistic given the hot plasma phase involved during the synthesis. For that purpose, we perform depth profiling chemical analysis of gold NPs produced by PLAL by varying the probing XPS photon energy. The relative proportion of signal from Au 4f and Br 3d informs on the NP chemical composition through a careful comparison with calculations based on the SESSA code.
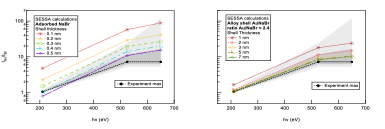
Figure 4: Experimental and SESSA calculations comparison using a NaBr shell model (left) or NaBr NP doping model (right).
PLAL colloids as plasmonic photosensitizers?
This research project also aims to evaluate the potential of PLAL NPs for energy harvesting applications as plasmonic photosensitizers. To increase the light absorption of semiconductors, heterojunctions including plasmonic materials are considered thanks to their ability to efficiently absorb visible light by excitation of plasmon waves. In the case of nanostructures, the plasmon resonance can be tuned by choosing the proper material and size and the photocurrent is no longer limited by the semiconductor band gap. One of the pathway to increase the electron injection efficiency in such heterojunction is based on the excitation of hot electrons in the NP that will be able to overcome the potential barrier (Schottky barrier) at the metal/semiconductor interface. However, such energy harvesting process is limited by different inherent factors which mainly reduce the number of electrons effectively injected into the semiconductor. The present project aims to investigate the potential of PLAL nanoparticles to optimize the injection efficiency using the following strategies, XPS and KPFM (Kelvin probe force microscopy).
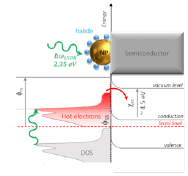
Figure 5: Hot electron injection on a metal/semiconductor heterojunction. One ultimate goal is to be able to control the Schottky barrier fSB.
- “Engineering the nanoparticle work function”. Adjusting the Schottky barrier height, fSB, could optimize the electron injection efficiency by reaching a favourable working point corresponding to the optimal condition where the energy required to cross the potential barrier is reduced for the hot electron population while keeping the return current due to thermionic emission to a reasonable value. Such optimal condition can be achieved by engineering the NP work function, fm, since, in a first approximation, fSB is function of fm and the semiconductor electron affinity, csc: fSB = fm – cSC. PLAL NPs are considered within this research project since the metallic work function can be modified by the adsorption of halide ions as evidences in the case of planar surfaces.
- “Engineering the nanoparticle density of states”. The density of states (DOS) of the plasmonic material has an important influence on the electron injection efficiency. PLAL synthesis enables the production of NPs of any kind of materials including alloys. The objective is to evaluate the potential of PLAL model NPs by a precise DOS engineering and characterization of different kind of NPs.
Collaborations
- INSP : équipes Physico-chimie et dynamique des surfaces (O. Pluchery) et Oxydes en basses dimensions (G. Cabailh)
- M. Patanen, Oulu University, Finlande;
- J. Gaudin et V. Blanchet, laboratoire CELIA, Bordeaux, France.;
- D. Amans et P. Mignon, laboratoire ILM , Lyon, France.
Publications
- Manuel de Anda Villa, Jérôme Gaudin, David Amans, Fahima Boudjada, John Bozek, et al.. Assessing the Surface Oxidation State of Free-Standing Gold Nanoparticles Produced by Laser Ablation. Langmuir, American Chemical Society, 2019, 35 (36), pp.11859-11871. ⟨10.1021/acs.langmuir.9b02159⟩. ⟨hal-02326073⟩
- Martino Trassinelli. Bayesian data analysis tools for atomic physics. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, Elsevier, 2017, 408, pp.301-312. ⟨10.1016/j.nimb.2017.05.030⟩. ⟨hal-01405864v2⟩