Impact of quantum coherences on the isotopic effect during ultrafast proton transfer
 Proton transfer caused by light excitation in small molecules represents an ideal platform for testing the limits of our understanding of many quantum phenomena. The ultrafast and inherently non-equilibrium dynamics between nuclei and electrons requires a purely quantum treatment, exponentially increasing the difficulty of numerical simulations. It is in this context that scientists from the INSP conducted an analysis of isotopic effect on proton transfer dynamics by comparing hydrogen and deuterium substitution under different light excitation conditions. This approach enabled detailed exploration of the effect of vibrations and electronic coherences on proton transfer dynamics.
Proton transfer caused by light excitation in small molecules represents an ideal platform for testing the limits of our understanding of many quantum phenomena. The ultrafast and inherently non-equilibrium dynamics between nuclei and electrons requires a purely quantum treatment, exponentially increasing the difficulty of numerical simulations. It is in this context that scientists from the INSP conducted an analysis of isotopic effect on proton transfer dynamics by comparing hydrogen and deuterium substitution under different light excitation conditions. This approach enabled detailed exploration of the effect of vibrations and electronic coherences on proton transfer dynamics.
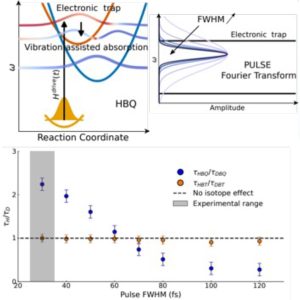
Figure: Dependence of the isotopic effect on pulse duration. One of the two studied systems shows an inversion of its isotopic effect for increasingly longer pulses. This phenomenon is explained by the dynamics of the wave packet trapped in HBQ for short pulses.
In this study, two reference molecules exhibiting ultrafast proton transfer (<100 fs), known as HBT and HBQ [1], were analyzed in depth along with their dynamics. Thanks to an innovative approach to simulating such phenomena based on the tensor formalism of matrix products, this study overcomes the numerical challenge associated with simulating a large quantum system. Unlike the predominantly static approaches of previous studies, our model integrates a dynamic description of the process with the explicit presence of 800 quantum vibrations, which are the main actors in both proton transfer and the associated electronic transformation. By replacing hydrogen with heavier deuterium, the possible difference in transfer time can indicate the role of the proton/deuteron, highlighted by the importance of the mass in the transfer. Conversely, identical transfer between the two isotopes indicates that vibrations enable the transfer during the temporal evolution after excitation.
The modification of excitation pulse characteristics reveals two opposing trends. On one side, remarkable electronic coherences enable ultrafast proton transfer. This highly efficient phenomenon illustrates how a system could allow optimized energy exchange. On the other side, and as represented in the Figure below, a trap state present in the energy landscape of one of the models is largely populated by brief pulses. Longer excitations, on the contrary, do not spectrally cover this state, and simultaneously reveal a vibration-assisted absorption phenomenon, examined notably through vibrational spectrum measurements.
In conclusion, the detailed analysis of two typical molecules for the study of excitedstate proton transfer has enabled extensive characterization of their respective dynamics. Furthermore, the isotopic effect of one of the two systems can be entirely controlled by the pulse, highlighting an experimental parameter that could be used in the future to study such systems. This analysis paves the way for the widespread use of an innovative method that takes into account all quantum effects for multidimensional systems.
[1] HBT : 2-(2’-hydroxyphenyl)benzothiazole , HBQ : 10-hydroxybenzo[h]quinoline. These molecules are ideal systems for the comparison between experiment and theory.
Reference :
« Impact and Interplay of Quantum Coherence and Dissipative Dynamics for Isotope Effects in Excited-State Intramolecular Proton Transfer »
Le Dé, Brieuc ; Huppert, Simon ; Spezia, Riccardo ; Chin, Alex W.
The Journal of Physical Chemistry Letters, 16, 10, 2514-2521 (2025)
https://pubs.acs.org/doi/abs/10.1021/acs.jpclett.4c03665
Contact : alex.chin@insp.jussieu.fr